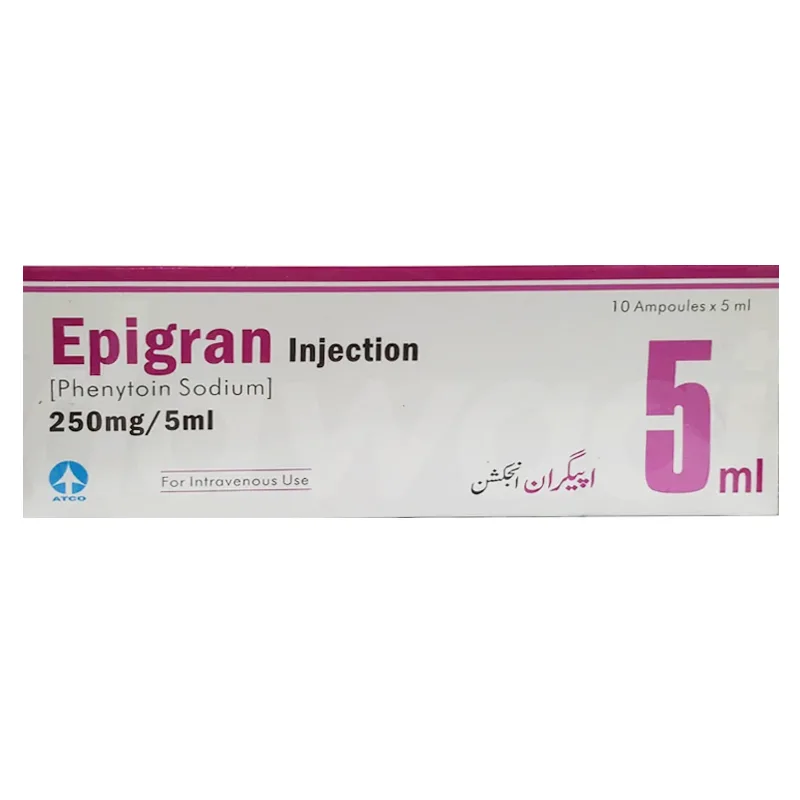
Epigran Injection 250mg/5ml
₨2,675.00 Original price was: ₨2,675.00.₨2,670.00Current price is: ₨2,670.00.
Epigran Injection (Phenytoin Sodium 250 mg/5 ml) is an anticonvulsant used for the treatment of status epilepticus and seizure prevention during neurosurgery. Manufactured by ATCO Laboratories, it stabilizes nerve activity by inhibiting abnormal electrical signals in the brain. Administered intravenously, it requires careful monitoring to avoid side effects like dizziness, nausea, and cardiovascular risks. Consult a doctor for proper dosage and safety precautions.
Epigran Injection is a pharmaceutical formulation containing Phenytoin Sodium, a well-established anticonvulsant used primarily for the management of various seizure disorders. Manufactured by ATCO Laboratories (Pvt) Ltd., Epigran Injection is designed to provide rapid control of seizures, particularly in acute settings.
Key Features:
- Active Ingredient: Phenytoin Sodium
- Strength: 250 mg per 5 ml ampoule
- Formulation: Sterile injectable solution
Manufacturer:
ATCO Laboratories (Pvt) Ltd. is a renowned pharmaceutical company committed to producing high-quality medications across various therapeutic categories. Their dedication to excellence ensures that Epigran Injection meets stringent quality standards.
Uses and Indications:
- Control of Status Epilepticus: Epigran Injection is indicated for the management of status epilepticus of the grand mal type, a life-threatening condition characterized by prolonged or repeated seizures without full recovery between episodes.
- Seizure Prevention During Neurosurgery: It is also utilized for the prevention and treatment of seizures that may occur during neurosurgical procedures, thereby enhancing patient safety during and after surgery.
Mechanism of Action:
Phenytoin Sodium, the active component of Epigran Injection, stabilizes neuronal membranes and decreases seizure activity by increasing efflux or decreasing influx of sodium ions across cell membranes in the motor cortex during generation of nerve impulses. This action helps to control the spread of seizure activity without affecting normal neuronal function.
Dosage and Administration:
- Status Epilepticus: The typical loading dose is 10 to 15 mg/kg administered intravenously at a rate not exceeding 50 mg per minute in adults. This is followed by maintenance doses of 100 mg orally or intravenously every 6 to 8 hours.
- Seizure Prevention During Neurosurgery: A loading dose of 10 to 15 mg/kg intravenously, followed by maintenance doses of 100 mg every 6 to 8 hours, is recommended.
Administration should be performed under close medical supervision with monitoring of cardiac function, blood pressure, and respiratory status due to the risk of cardiovascular complications.
Side Effects:
Common side effects may include:
- Nystagmus (involuntary eye movement)
- Ataxia (lack of muscle coordination)
- Slurred speech
- Dizziness
- Nausea and vomiting
- Rash
Serious adverse effects, though rare, can occur and may include severe skin reactions (e.g., Stevens-Johnson syndrome), hepatotoxicity, hematologic disorders, and cardiovascular complications such as hypotension and arrhythmias. Immediate medical attention is warranted if severe reactions are observed.
Precautions and Warnings:
- Cardiovascular Risk: Intravenous administration should be done cautiously, monitoring for potential cardiovascular adverse effects.
- Hypersensitivity: Patients with a history of hypersensitivity to phenytoin or other hydantoins should avoid using this medication.
- Hepatic Impairment: Use with caution in patients with liver dysfunction, as phenytoin is metabolized hepatically.
- Pregnancy and Lactation: Phenytoin is classified as a pregnancy category D drug, indicating potential risk to the fetus. It should be used during pregnancy only if the potential benefits justify the risks. Phenytoin is excreted in breast milk; therefore, caution is advised when administering to breastfeeding mothers.
Frequently Asked Questions:
- What is the price of Epigran Injection in Pakistan?
The price of Epigran Injection 250 mg per 5 ml ampoule may vary depending on the pharmacy and location. For the most current pricing, it is advisable to consult local pharmacies or authorized medical distributors.
- How is Epigran Injection administered?
Epigran Injection is administered intravenously under the supervision of a qualified healthcare professional. The dosage and rate of administration depend on the clinical condition being treated and the patient’s individual response.
- What are the common side effects of Epigran Injection?
Common side effects include involuntary eye movements, lack of muscle coordination, slurred speech, dizziness, nausea, vomiting, and skin rash. Patients should report any adverse effects to their healthcare provider promptly.
- Can Epigran Injection be used during pregnancy?
Epigran Injection should be used during pregnancy only if the potential benefits outweigh the risks to the fetus. It is essential to consult a healthcare provider to discuss the risks and benefits before using this medication during pregnancy.
- Are there any dietary restrictions while receiving Epigran Injection?
There are no specific dietary restrictions associated with Epigran Injection. However, maintaining a balanced diet and avoiding alcohol is advisable, as alcohol can alter phenytoin levels and increase the risk of side effects.
Relevant Product Tags:
Epigran Injection, Phenytoin Sodium Injection, Anticonvulsant Injection, Status Epilepticus Treatment, Seizure Management, ATCO Laboratories, Epigran Injection Price in Pakistan, Epigran Injection Side Effects, Epigran Injection Dosage
Please note that this information is provided for educational purposes and should not replace professional medical advice. Always consult a healthcare provider for medical guidance tailored to your health condition.
| Weight | 0.5 kg |
|---|




🚚 Shipping & Delivery Partners
At MedicineWalyDost.com, we deliver medicines through trusted courier services including TCS, Leopard Courier, Daewoo Fastex, and inDrive to ensure safe, timely, and reliable delivery across Pakistan.
The courier partner for each order is carefully selected based on:
Customer location and city coverage
Delivery speed and route availability
Nature of the product (sensitivity, urgency, or handling requirements)
This approach allows us to use the most suitable courier for every order, ensuring your medicine reaches you in the best possible condition.
📦 Secure & Discreet Packaging
All medicines are packed securely with proper protection and confidential packaging to maintain privacy during transit.
⏱ Delivery Timeline
Orders are usually dispatched within 1–2 working days after payment confirmation
Delivery typically takes 2–3 working days, depending on location and courier service
📍 Nationwide Coverage
With multiple courier partners, we provide wide coverage across Pakistan, including major cities and selected remote areas.
Once your order is dispatched, tracking details are shared for your convenience.